Class 2 Drug Alert
Published: 22/06/21

Bristol Laboratories Limited, Brown & Burk UK Ltd and Teva UK Ltd are recalling the below batches of products as a precautionary measure due to contamination with an impurity.
The impurity is called 5-(4’-(azidomethyl)- [1,1’-biphenyl]-2yl)-1H-tetrazole, which has mutagenic potential. These batches are being recalled as the level of contamination is above the acceptable limit.
Stop supplying the above batches immediately. Quarantine all remaining stock and return it to your supplier using your supplier’s approved process.
Patients are advised not to stop taking their medication prior to consultation with their doctor or pharmacist, as the health risk of discontinuing the medicine is higher than the potential risk presented by the impurity. This recall is being undertaken as a precautionary measure to prevent further exposure to this impurity in the affected medicines whilst the investigation is ongoing.
There are no anticipated shortages of Irbesartan-containing and losartan-containing products in the UK as a result of this recall. However, this is a developing issue and MHRA is working with Marketing Authorisation Holders and other medicines regulators to determine any possible impact. An investigation into other potentially impacted products is continuing and further updates will be provided as the investigation progresses.
Endometriosis Awareness Month runs throughout March, raising awareness of symptoms and encouraging women to seek medical advice.
Pharmacist Support launches the 2026 Student ACTNow campaign, urging pharmacy students to prioritise mental health on University Mental Health Day.
Experienced Pharmacist role at Saltcoats Health Centre. Deliver clinical services in a busy community pharmacy setting.
This is an overview of the key policy activity, engagement and progress taking place to support and represent the community pharmacy network.
This message is for Independent Prescribing colleagues who are funded by Community Pharmacy Scotland (CPS) and NHS Education for Scotland (NES):
Senior Pharmacy Manager and Independent Prescriber role in Stevenston. Lead a busy community pharmacy with strong clinical focus.
Why the 2026 Scottish Parliamentary Election Matters for Community Pharmacy and What CPS Is Doing About It.
Ahead of the 2026 Scottish Parliamentary elections, Community Pharmacy Scotland (CPS) has identified four key areas
This week’s MCR Ready Reckoner has been updated and is now available to download or you can view it via the App.
Guidelines for your prescription courier collection on Thursday, 5th March 2026, including bag usage, required forms and contact details.
An update on March 2026 Part 7 publishing timelines following early finalisation of February adjusted prices. Key dates explained.
PVG membership is now a legal requirement for locum pharmacists in Scotland. Learn what this means and how to apply.
Join Boots Barrhead as a part‑time Pharmacy Technician. Flexible hours, supportive team, checking role, and great benefits.
Join Boots Motherwell as a Pharmacy Technician. Work in a modern, supportive pharmacy with great development and benefits.
Join Boots as a Relief Pharmacist in West Lothian. Enjoy flexible hours, varied locations, strong support, and great benefits.
Join Boots Hamilton as an Independent Prescribing Pharmacist. Shape pharmacy practice with strong team support and great benefits.
Join Boots Largs as a Pharmacy Technician. Work in a supportive store offering Pharmacy First Plus with great benefits and development.
Join Boots Kelso as a Pharmacy Technician. Work in a supportive, community‑focused pharmacy with great benefits and development.
Pharmacy teams will start to notice two new UCF modules appearing in their PMRs from next week - one each for the previously-announced Nystatin and Hydrocortisone PGDs.
Join Boots Glenrothes as an experienced Pharmacist. Work in a high‑performing pharmacy with strong GP links and excellent benefits.
Join Boots Dundee as a Pharmacy Technician. Work in a busy community pharmacy with great support, development, and benefits.
Join Boots Stranraer as a Pharmacist. Work in a well‑supported store with strong leadership, great benefits, and coastal living.
Join Boots Kelso as a Pharmacist. Work in a supportive, community‑focused pharmacy with strong GP links and excellent benefits.
Join Boots Kirkcaldy as a full‑time Pharmacist. Work with a supportive team, deliver excellent care, and enjoy great benefits.
Join Boots Dundee as a Pharmacist. Work with a fantastic team, modern automation, and strong community links with excellent benefits.
Join Boots in Forfar as a Relief Pharmacist. Enjoy flexibility, variety, great benefits, and the chance to make a real community impact.
Join Boots in Oban as an Independent Prescribing Pharmacist. Build your clinical career on Scotland’s west coast with great benefits.
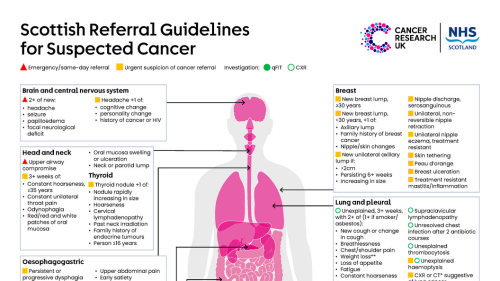
Our colleagues at Cancer Research UK and Healthcare Improvement Scotland have highlighted a useful resource for community pharmacy teams.
Our colleagues at NHS Education for Scotland (NES) have asked us to highlight the Behavioural Change in Healthcare Webinar on Wednesday 18th March 2026 at 7pm.
Digital Communications Officer















Lead a new community pharmacy in Bishopton as Pharmacist Manager. Competitive salary, GPhC fees paid and opportunity to shape services.