Guidance on Puberty Blockers for Prescribers & Dispensers
Published: 29/05/25

The Scottish Government has issued an update to prescribers and pharmacists on the use and supply of puberty suppressing hormones.
Following a targeted consultation and advice from the Commission on Human Medicines (CHM), the UK Government laid legislation on 11 December 2024, which came into effect on 01 January 2025, that put in place an indefinite ban on the use of gonadotrophin releasing hormone (GnRH) analogues when prescribed to suppress puberty as part of treating gender incongruence and/or gender dysphoria in children and young people who are under 18 years of age.
The following update gives additional information to pharmacists on the arrangements that apply to medicines that consist of or contain buserelin, gonadorelin, goserelin, leuprorelin acetate, nafarelin, or triptorelin. This includes, but is not limited to, medicines sold under the brand names: Decapeptyl®, Gonapeptyl Depot®, Salvacyl®, Prostap®, Staladex®, Zoladex®, Synarel®.
Please take the time to read through the information and ensure you are up-to-date with current legislation relating to this topic.
If you have any questions, please contact us at enquiries@cps.scot.
PVG membership is now a legal requirement for locum pharmacists in Scotland. Learn what this means and how to apply.
Join Boots Barrhead as a part‑time Pharmacy Technician. Flexible hours, supportive team, checking role, and great benefits.
Join Boots Motherwell as a Pharmacy Technician. Work in a modern, supportive pharmacy with great development and benefits.
Join Boots as a Relief Pharmacist in West Lothian. Enjoy flexible hours, varied locations, strong support, and great benefits.
Join Boots Hamilton as an Independent Prescribing Pharmacist. Shape pharmacy practice with strong team support and great benefits.
Join Boots Largs as a Pharmacy Technician. Work in a supportive store offering Pharmacy First Plus with great benefits and development.
Join Boots Kelso as a Pharmacy Technician. Work in a supportive, community‑focused pharmacy with great benefits and development.
Pharmacy teams will start to notice two new UCF modules appearing in their PMRs from next week - one each for the previously-announced Nystatin and Hydrocortisone PGDs.
Join Boots Glenrothes as an experienced Pharmacist. Work in a high‑performing pharmacy with strong GP links and excellent benefits.
Join Boots Dundee as a Pharmacy Technician. Work in a busy community pharmacy with great support, development, and benefits.
Join Boots Stranraer as a Pharmacist. Work in a well‑supported store with strong leadership, great benefits, and coastal living.
Join Boots Kelso as a Pharmacist. Work in a supportive, community‑focused pharmacy with strong GP links and excellent benefits.
Join Boots Kirkcaldy as a full‑time Pharmacist. Work with a supportive team, deliver excellent care, and enjoy great benefits.
Join Boots Dundee as a Pharmacist. Work with a fantastic team, modern automation, and strong community links with excellent benefits.
Join Boots in Forfar as a Relief Pharmacist. Enjoy flexibility, variety, great benefits, and the chance to make a real community impact.
Join Boots in Oban as an Independent Prescribing Pharmacist. Build your clinical career on Scotland’s west coast with great benefits.
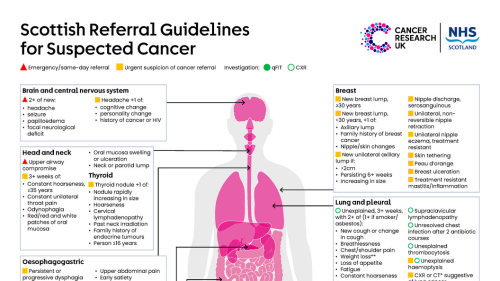
Our colleagues at Cancer Research UK and Healthcare Improvement Scotland have highlighted a useful resource for community pharmacy teams.
Our colleagues at NHS Education for Scotland (NES) have asked us to highlight the Behavioural Change in Healthcare Webinar on Wednesday 18th March 2026 at 7pm.
As of 24 February, the ReThink Dementia campaign is now running. You should have these posters displayed in your pharmacy.
Last Friday, the Community Pharmacy Scotland (CPS) Policy team attended both the Scottish Conservative and Scottish Liberal Democrat party conferences.
This week’s MCR Ready Reckoner has been updated and is now available to download or you can view it via the App.
Check out our Policy & Development Officer, Gordon Winter, speaking to BBC Radio Scotland yesterday about the shortage of prescription-strength co-codamol.
Learn how the Nip It In The Bud campaign supports early cancer detection in farming and rural communities through awareness and community pharmacy support.
Read our response to the GPhC consultation on draft rules and standards for Responsible and Superintendent Pharmacists, following legislative changes in 2022.
Your next courier collection is scheduled for Thursday, 19th February. We have produced the following guidelines for your prescription bag submissions.
Join Davidsons Chemists in Newburgh, Fife as Pharmacist Manager. Lead a community‑focused team with excellent support, benefits, and development.
Join M&D Green Oban as a Pharmacist Manager. Lead a strong team, support patient care, and enjoy excellent benefits. Apply today.
Join M&D Green Stranraer as a Pharmacist Manager. Lead a great team, develop your career, and enjoy excellent benefits. Apply today.
The following are the Top Three items currently under discussion and review by your CPS Board. There is no specific detail, yet, as these are currently “works in progress”. Further detail will follow as available.














An update on March 2026 Part 7 publishing timelines following early finalisation of February adjusted prices. Key dates explained.